Rally #220-7-1025BX
NDC #0006-4681-00
Merck - M-M-R® II Measles, Mumps, and Rubella Vaccine (10 Vials / Box)
Suspension For Intramuscular or Subcutaneous Injection, Lyophilized, Single-Dose Vial, 0.5-mL, No Preservative, Rx Only
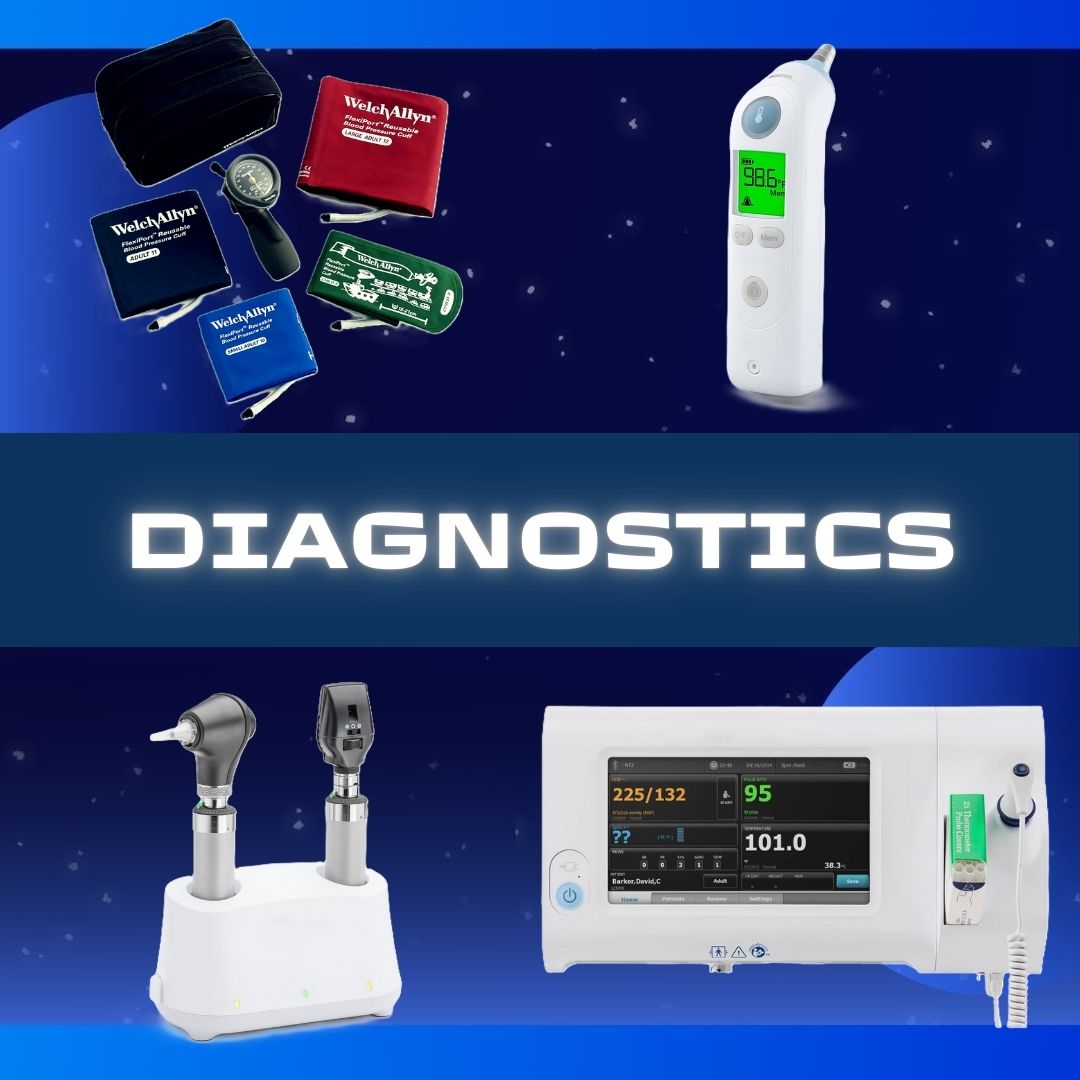
Features
- Lyophilized
- No preservative
- Can be use on indivduals 12 months of age and older
- Provides coverage that can last over 10 years
M-M-R®II is contraindicated in certain individuals, including those with: a history of hypersensitivity to any component of the vaccine, including gelatin; a history of anaphylactic reaction to neomycin; individuals who are immunodeficient or immunosuppressed due to disease or medical therapy; an active febrile illness; active untreated tuberculosis; or those who are pregnant or are planning to become pregnant within the next month.
This product contains a box of 10 single-dose vials of lyophilized vaccine.
Important Note: Product is Perishable and Non-Returnable. This product requires Refrigeration for storage. DO NOT FREEZE. Due to safety and perishability precautions, product will not be shipped on Fridays. Please take all necessary precautions before ordering this product. As this product is non-returnable, please confirm it is the right product before ordering.
Please see Education and Documents section for Prescribing Information.
| Rally # | 220-7-1025BX |
| NDC# | 0006-4681-00 |
| Brand | M-M-R® II |
| Manufacturer | Merck |
| Country of Origin | United States |
| Application | MMR Vaccine |
| Generic Drug Name | Measles, Mumps, and Rubella Vaccine |
| Type | Intravenous or Subcutaneous |
| Container Type | Single-Dose Vial |
| Dosage Form | Injection |
| Storage Requirements | Requires Refrigeration |
| Strength | 1,000 TCID - 12,500 TCID - 1,000 TCID / 0.5 mL |
| Volume | 0.5 mL |
| UNSPSC Code | 51201628 |
| User | Indicated for People 12 Months of Age and Older |
| Preservatives | Preservative Free |
| Initial U.S. Approval | 1978 |
| Storage Temperature | See Storage and Handling in Highlights |
These highlights do not include all the information needed to use M-M-R II safely and effectively.
See full prescribing information for M-M-R II
here.
M-M-R® II (Measles, Mumps, and Rubella Virus Vaccine Live)
Suspension for Intramuscular or Subcutaneous Injection
Initial U.S. Approval: 1978
M-M-R II is a vaccine indicated for active immunization for the prevention of measles, mumps, and rubella in individuals 12 months of age and older.
For intramuscular or subcutaneous injection only.
A single dose is approximately 0.5 mL.
- The first dose is administered at 12 to 15 months of age.
- The second dose is administered at 4 to 6 years of age.
Suspension for injection (approximately 0.5 mL dose) supplied as a lyophilized vaccine to be reconstituted using accompanying sterile diluent.
- Hypersensitivity to any component of the vaccine.
- Immunosuppression.
- Moderate or severe febrile illness.
- Active untreated tuberculosis.
- Pregnancy.
- Use caution when administering M-M-R II to individuals with a history of febrile seizures.
- Use caution when administering M-M-R II to individuals with anaphylaxis or immediate hypersensitivity following egg ingestion.
- Use caution when administering M-M-R II to individuals with a history of thrombocytopenia.
- Evaluate individuals for immune competence prior to administration of M-M-R II if there is a family history of congenital or hereditary immunodeficiency.
- Immune Globulins (IG) and other blood products should not be given concurrently with M-M-R II.
See full prescribing information for adverse reactions occurring during clinical trials or the post-marketing period.
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
- Administration of immune globulins and other blood products concurrently with M-M-R II vaccine may interfere with the expected immune response.
- M-M-R II vaccination may result in a temporary depression of purified protein derivative (PPD) tuberculin skin sensitivity.
Pregnancy: Do not administer M-M-R II to females who are pregnant. Pregnancy should be avoided for 1 month following vaccination with M-M-R II.
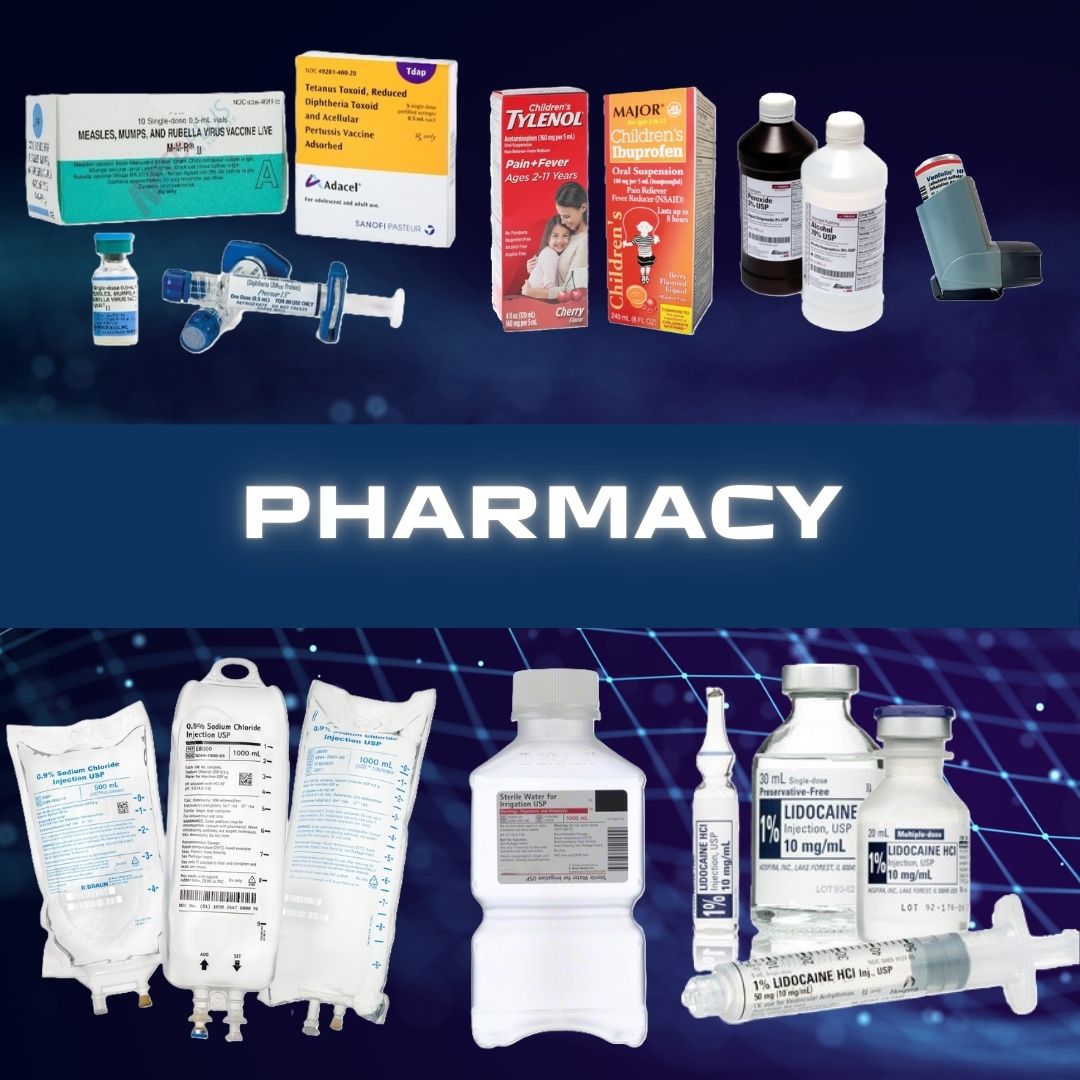
M-M-R II vaccine is a sterile lyophilized preparation of (1) Measles Virus Vaccine Live, an attenuated line of measles virus, derived from Enders' attenuated Edmonston strain and propagated in chick embryo cell culture; (2) Mumps Virus Vaccine Live, the Jeryl Lynn™ (B level) strain of mumps virus propagated in chick embryo cell culture; and (3) Rubella Virus Vaccine Live, the Wistar RA 27/3 strain of live attenuated rubella virus propagated in WI-38 human diploid lung fibroblasts. The cells, virus pools, recombinant human serum albumin and fetal bovine serum used in manufacturing are tested and determined to be free of adventitious agents.
After reconstitution, each approximately 0.5 mL dose contains not less than 3.0 log10 TCID50 (tissue culture infectious doses) of measles virus; 4.1 log10 TCID50 of mumps virus; and 3.0 log10 TCID50 of rubella virus.
Each dose is calculated to contain sorbitol (14.5 mg), sucrose (1.9 mg), hydrolyzed gelatin (14.5 mg), recombinant human albumin (≤0.3 mg), fetal bovine serum (<1 ppm), approximately 25 mcg of neomycin and other buffer and media ingredients. The product contains no preservative.
M-M-R II vaccine is supplied as follows:
(1) a box of 10 single-dose vials of lyophilized vaccine (package A), NDC 0006-4681-00
(2) a box of 10 prefilled syringes of sterile diluent, NDC 0006-4175-88 (package B) OR a box of 10 vials of sterile diluent, NDC 0006-4309-00 (package B)
- Vaccine Vial -
Exposure to light may inactivate the vaccine viruses.
To maintain potency, M-M-R II must be stored between -58°F and +46°F (-50°C to +8°C). Use of dry ice may subject M-M-R II to temperatures colder than -58°F (-50°C).
Before reconstitution, refrigerate the lyophilized vaccine at 36°F to 46°F (2°C to 8°C).
- Sterile Diluent -
The sterile diluent should be stored in the refrigerator (36°F to 46°F, 2°C to 8°C) or at room temperature (68°F to 77°F, 20°C to 25°C). Do not freeze the sterile diluent.
Administer M-M-R II vaccine immediately after reconstitution. If not administered immediately, reconstituted vaccine may be stored between 36°F to 46°F (2°C to 8°C), protected from light, for up to 8 hours. Discard reconstituted vaccine if it is not used within 8 hours.
For information regarding the product or questions regarding storage conditions, call 1-800-637-2590.
Currently Unavailable
Documentation Links
Reimbursements Information
This product maybe reimbursable, depending on various factors including current law and policies. Rally Inc. does not handle these reimbursements; instead, each reimbursement is handled solely between you, the customer, and the manufacturer, Merck & Co., Inc.. Rally Inc. does not oversee these reimbursements. To check eligibility and get your reimbursements, please follow the manufacturer's reimbursements instructions.
You can find more informations on reimbursement details here.
Size: 0.5 mL