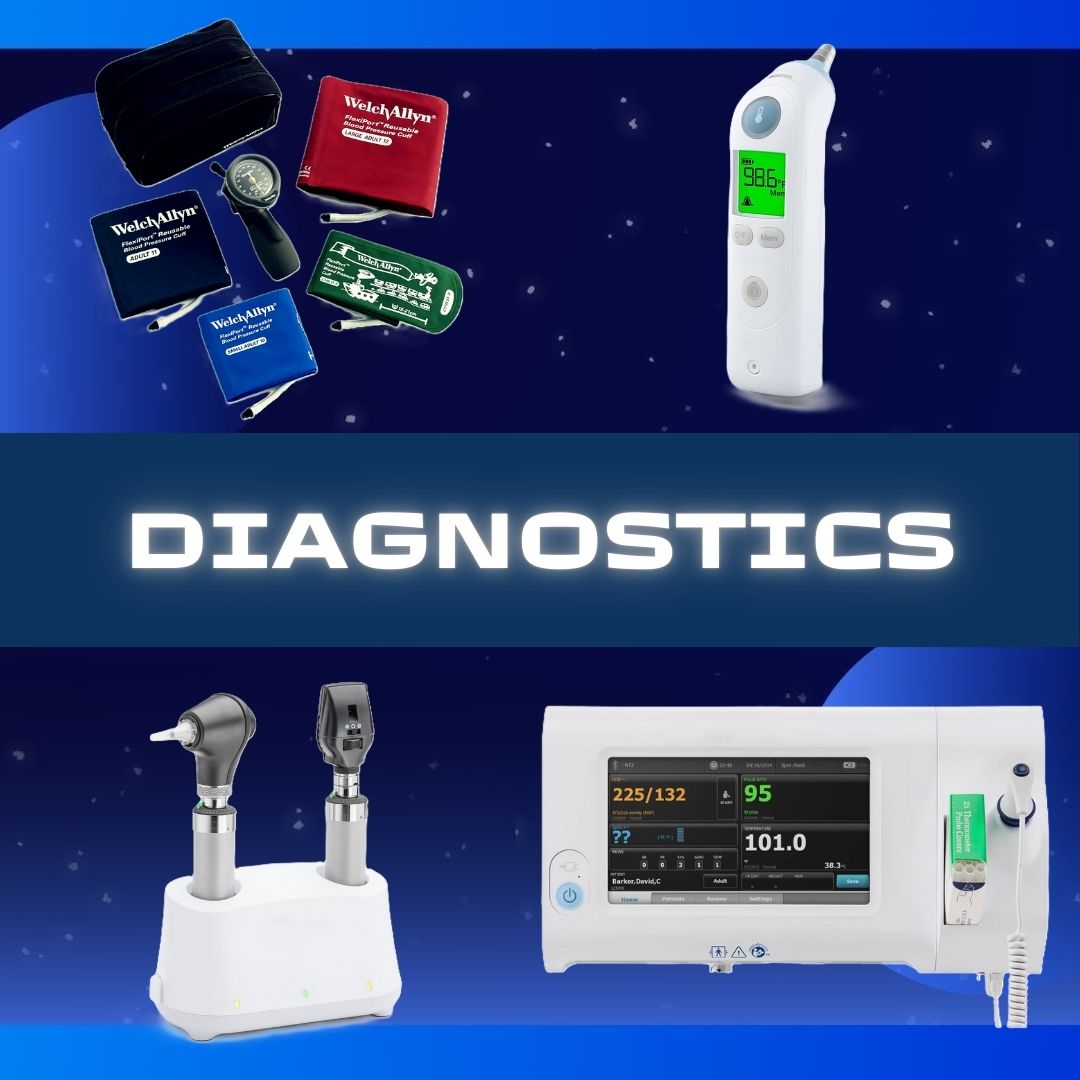
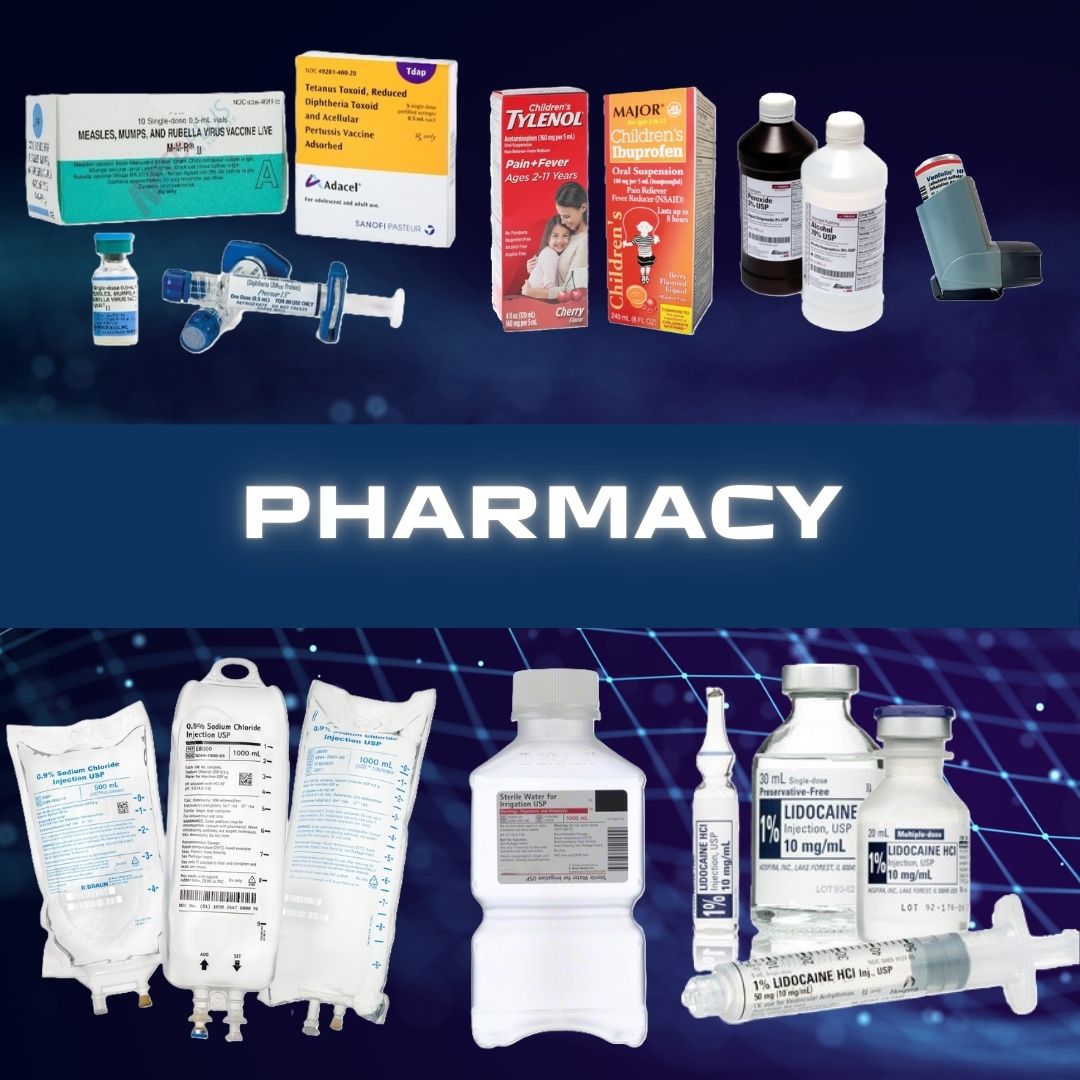
Hospira #00409488810 Diluent Sodium Chloride, Preservative Free 0.9% Intramuscular, Intravenous, or Subcutaneous Solution Single Dose Vial 10 mL SODIUM CHLORIDE, VL PF 0.9% 10ML (25/PK) Flip-top plastic vial This parenteral preparation is indicated only for diluting or dissolving drugs for intravenous, intramuscular or subcutaneous injection, according to instructions of the manufacturer of the drug to be administered The semi-rigid vial is fabricated from a specially formulated polyolefin Manufacturer # 00409488810 Manufacturer Hospira Country of Origin Unknown Application Diluent Container Type Single Dose Vial Dosage Form Solution Generic Drug Code 03034 Generic Drug Name Sodium Chloride, Preservative Free HCPCS A4218 NDC Number 00409-4888-10 Product Dating Acceptable Dating: we will ship >= 90 days Storage Requirements USP Controlled Room Temperature Strength 0.9% Type Intramuscular, Intravenous, or Subcutaneous UNSPSC Code 51191602 Volume 10 mL Latex Free Indicator Not Made with Natural Rubber Latex
Size: 10ML
More Information