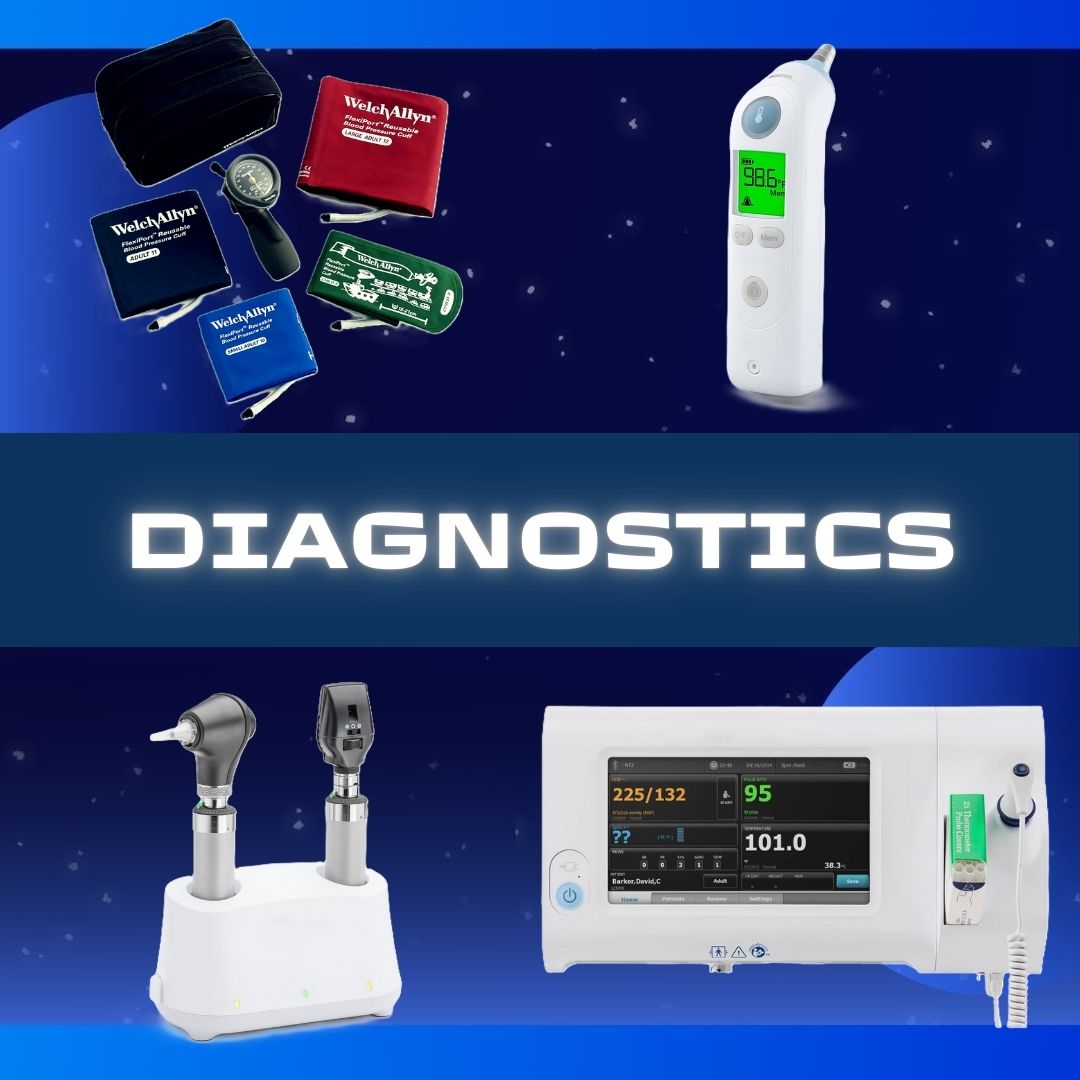
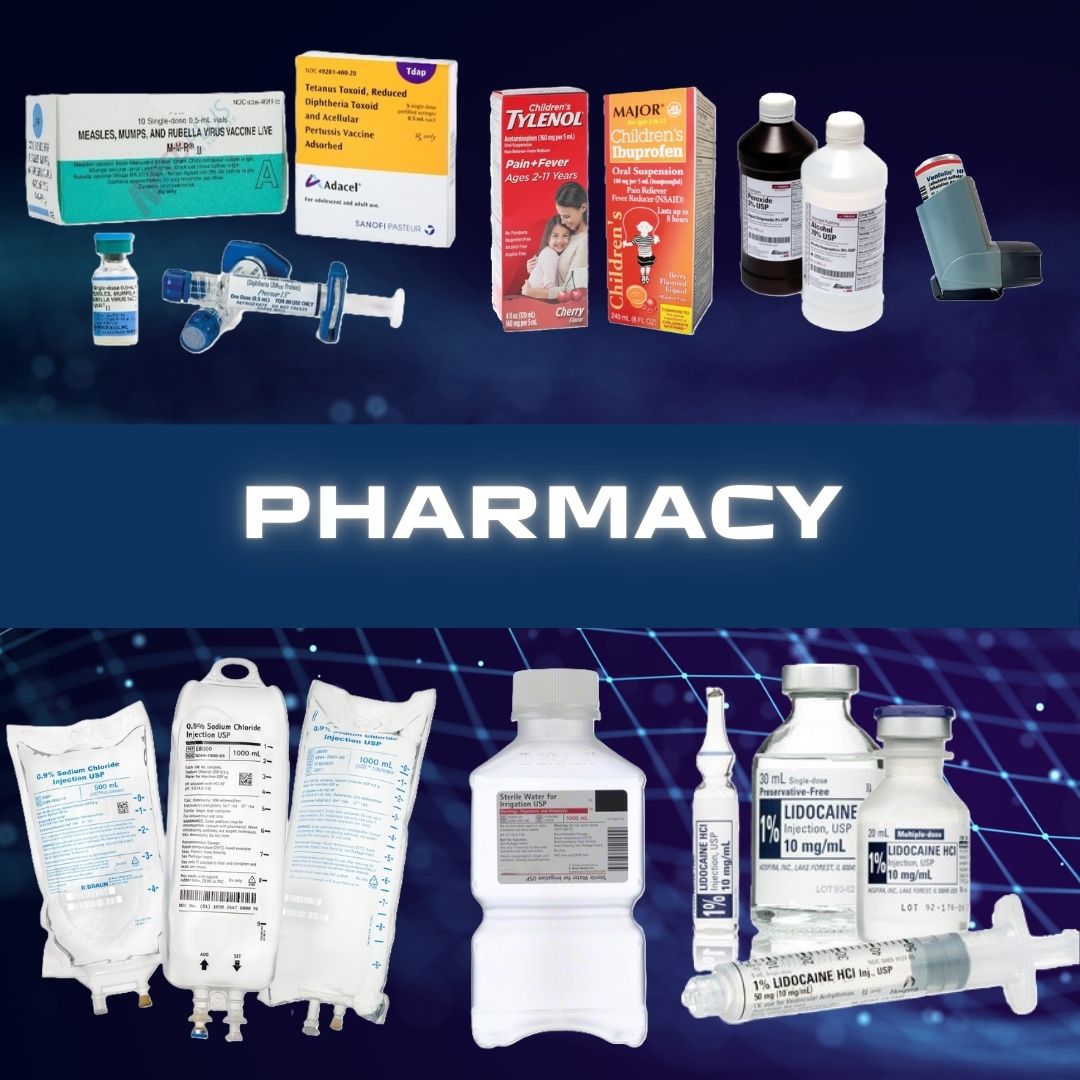
APOTEX NDC Number : 60505-6146-04 Material Number : 65052 Active Ingredient : Cefepime Bioequivalent to * : MAXIPIME® Therapeutic Class (AHFS) : Fourth Generation Cephalospori Strength : 1G Pack Size (Form) : 10X20ML INJECTABLE (VIAL) Dosage Form : Powder Scored : N/A Color : White to pale yellow powder Shape : N/A Markings : N/A Route of Administration : Intravenous Storage Condition : Controlled Room Temperature (68 - 77 degrees F) Minimum Order Quantity : 1 Multiple Order Quantity : 1 Latex Free : Yes Sugar Free : Yes Dye Free : Yes Alcohol Free : Yes Preservative Free : Yes Hazardous Material : No - INDICATIONS AND USAGE ----------- Cefepime for Injection is a cephalosporin antibacterial indicated for the treatment of the following infections caused by susceptible strains of the designated microorganisms: Pneumonia. (1.1) Empiric therapy for febrile neutropenic patients. (1.2) Uncomplicated and complicated urinary tract infections (including pyelonephritis). (1.3) Uncomplicated skin and skin structure infections. (1.4) Complicated intra-abdominal infections (used in combination with metronidazole) in adults.(1.5) To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefepime for Injection and other antibacterial drugs, Cefepime for Injection should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. (1.6) DOSAGE FORMS AND STRENGTHS---------- Cefepime for Injection is a sterile powder of cefepime in vials for reconstitution, available in the following strengths: 0.5 grams per vial, 1 gram per vial and 2 grams per vial. (3) To report SUSPECTED ADVERSE REACTIONS, contact Apotex Corp. at 1-800-706-5575, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Storage and Handling Cefepime for injection in the dry state should be stored at 20° to 25°C (68° to 77°F) [see USP controlled room temperature.] and protected from light. NDC 60505-6146-4 Carton containing 10
Size: 1G 20ML
More Information